Abstract
Introduction: Despite outstanding cure rates for newly diagnosed patients with acute lymphoblastic leukemia (ALL), outcomes for pediatric patients with relapsed or refractory (R/R) disease remain unsatisfactory. Inotuzumab ozogamicin (InO), a CD22-directed antibody-drug conjugate, has shown remarkable activity; however, predictors of response to InO beyond CD22 expression and site density have not yet been reported.
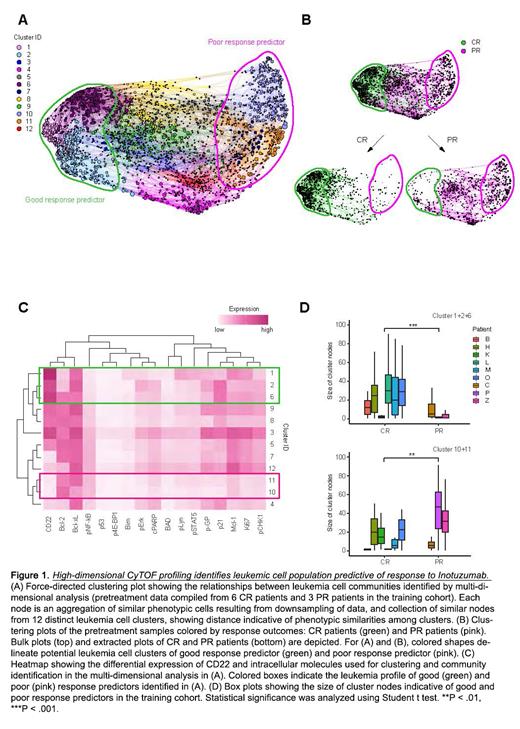
Methods: In order to identify better predictors of response to InO, we analyzed 68 samples from 28 patients with multiply R/R ALL enrolled in Children's Oncology Group trial AALL1621 (NCT02981628). Samples were collected before and after treatment with InO. We performed a B-ALL-centric protein profiling approach for which we designed a custom CyTOF panel, encompassing 35 rationally selected proteins (antigens) as potential predictors of InO treatment. Those proteins included surface markers (such as CD10/19/20/22 expressed in B cells and CD3/CD14, CD33 expressed in non B cells), intracellular signaling pathways (such as MAPK, PI3K, JAK/STATs), survival pathways (such as BCL-2 family members) and pathways reported to be involved in resistance to various treatments (such as p53/pCHK1 pathway, ABC drug efflux transporters, and NF-kB). This approach allowed measurement of protein levels of all those antigens. We then designed a high-dimensional tumor cell-community clustering algorithm providing dimensionality reduction to determine the extent of antigen expression across all patients at diagnosis and end of cycle 1 and cycle 2 of treatment.
Results: high-dimensional clustering analysis depicted distinct tumor profiles between complete responder and partial responder patient groups (Figure 1). While this clustering measured many antigens, those that contributed the most to identification of the groups were Bcl-xl, Bcl-2, p21, CD22, Mcl1, pCHK1 and pGP whereas those contributing the least were BAD, BIM, p4EBP1 and p53. Our analyses identified the presence of CD22 high cells and CD22 low/Bcl-2 high cells as predictors of good and poor response, respectively. Furthermore, analysis of residual leukemia cells at the end of cycle 1 or 2 showed persistent high expression of Bcl-2 family members.
Conclusions: We have shown for the first time that a novel strategy for multidimensional single cell phenotyping of lymphoblasts can reliably identify a predictive dual-marker signature with CD22 high expression predicting good response to InO versus CD22 low/Bcl-2 high expression predicting poor response. This approach also identifies dynamic changes of tumor composition following InO treatment. These data provide new insights into predictors of response to InO and suggest that Bcl-2 inhibition may have the potential for synergistic clinical efficacy, allowing for a potential patient-stratified approach for InO combinatorial treatments. Given that this experimental approach can be readily done in any laboratory equipped with flow or mass cytometry, we expect that our findings may be further replicated and validated in other studies using InO.
Acknowledgements
This work was supported by U10CA180886 and U10CA180899, the St. Baldrick's Foundation (MLL), a Pfizer ASPIRE award (MLL, EDF, AW, MO, SZ, AT), the German Cancer Aid (AW) and Uehara Memorial Foundation (KI). MLL is the Benioff Chair of Children's Health and the Deborah and Arthur Ablin Chair of Pediatric Molecular Oncology.
Wood: Juno, Pfizer, Amgen, Seattle Genetics: Other: Laboratory Services Agreement; Pfizer, Amgen, Seattle Genetics: Honoraria. O'Brien: Jazz: Honoraria; Pfizer: Honoraria, Research Funding. Loh: MediSix therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal